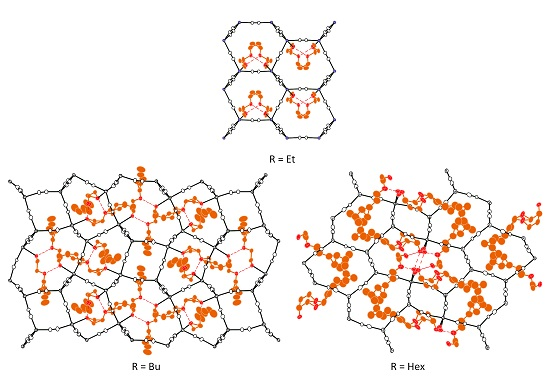
Crystals | Free Full-Text | Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol

Homologous Critical Behavior in the Molecular Frameworks Zn(CN)2 and Cd (imidazolate)2 | Journal of the American Chemical Society
![SOLVED:At what concentration of cyanide ion is (a) [Cd^2+]=10^-8 ×[Cd(CN)4^2-] ? (b) [Fe^2+]=10^-20 ×[Fe(CN)6^4-] ? SOLVED:At what concentration of cyanide ion is (a) [Cd^2+]=10^-8 ×[Cd(CN)4^2-] ? (b) [Fe^2+]=10^-20 ×[Fe(CN)6^4-] ?](https://cdn.numerade.com/previews/d20de7a7-e8b2-4edd-851a-0a4fed0b9d64_large.jpg)
SOLVED:At what concentration of cyanide ion is (a) [Cd^2+]=10^-8 ×[Cd(CN)4^2-] ? (b) [Fe^2+]=10^-20 ×[Fe(CN)6^4-] ?
![Synthesis, PtS-type structure, and anomalous mechanics of the Cd(CN)2 precursor Cd(NH3)2[Cd(CN)4] - Dalton Transactions (RSC Publishing) Synthesis, PtS-type structure, and anomalous mechanics of the Cd(CN)2 precursor Cd(NH3)2[Cd(CN)4] - Dalton Transactions (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C8DT01128A)
Synthesis, PtS-type structure, and anomalous mechanics of the Cd(CN)2 precursor Cd(NH3)2[Cd(CN)4] - Dalton Transactions (RSC Publishing)
![SOLVED: Formula Kf [Ag(CN)2] - 3.0x1020 [Aglz] 1.0x1011 [Ag(NH3)2] + 1.7x107 [Ag(SzO3)2]3 - 4.7*1013 [AlF6]3 - 4. 0x1019 [Au(CN)z] 2.0x1038 [Cd( CN)4]2 - 7.7x1016 [Co(NH3)6]3+ 4 6x1033 [Cu(CN)2] 1.0x1016 [Culz] 8.0x108 [Cu(NH3)4]2+ 5.6x1011 [ SOLVED: Formula Kf [Ag(CN)2] - 3.0x1020 [Aglz] 1.0x1011 [Ag(NH3)2] + 1.7x107 [Ag(SzO3)2]3 - 4.7*1013 [AlF6]3 - 4. 0x1019 [Au(CN)z] 2.0x1038 [Cd( CN)4]2 - 7.7x1016 [Co(NH3)6]3+ 4 6x1033 [Cu(CN)2] 1.0x1016 [Culz] 8.0x108 [Cu(NH3)4]2+ 5.6x1011 [](https://cdn.numerade.com/ask_images/73f4162e596a4bcdb226bde847a824a9.jpg)
SOLVED: Formula Kf [Ag(CN)2] - 3.0x1020 [Aglz] 1.0x1011 [Ag(NH3)2] + 1.7x107 [Ag(SzO3)2]3 - 4.7*1013 [AlF6]3 - 4. 0x1019 [Au(CN)z] 2.0x1038 [Cd( CN)4]2 - 7.7x1016 [Co(NH3)6]3+ 4 6x1033 [Cu(CN)2] 1.0x1016 [Culz] 8.0x108 [Cu(NH3)4]2+ 5.6x1011 [

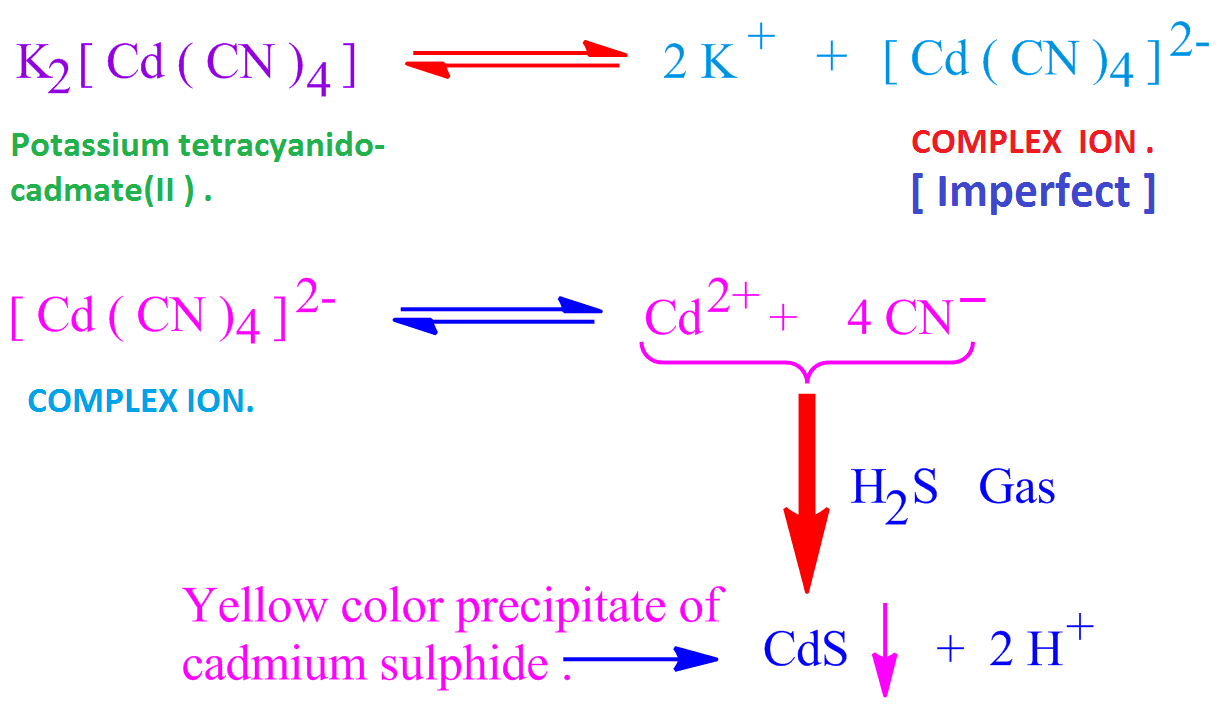
In the separation of Cu^2 + and Cd^2 + in second group of qualitative analysis of cations, tetraaminecopper (II) sulphate and tetraaminecadmium ( II) sulphate react with KCN to form corresponding cyano complexes
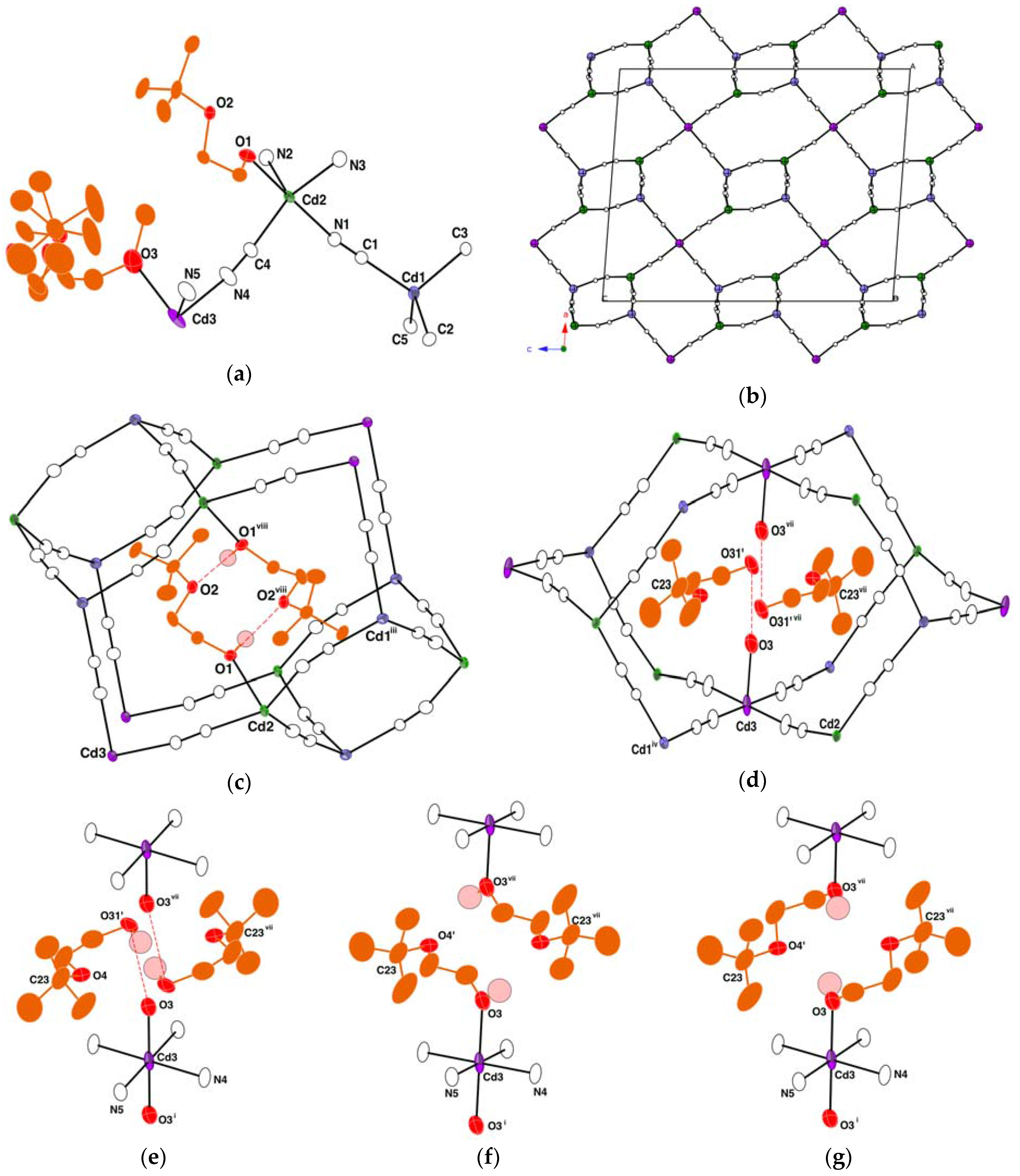
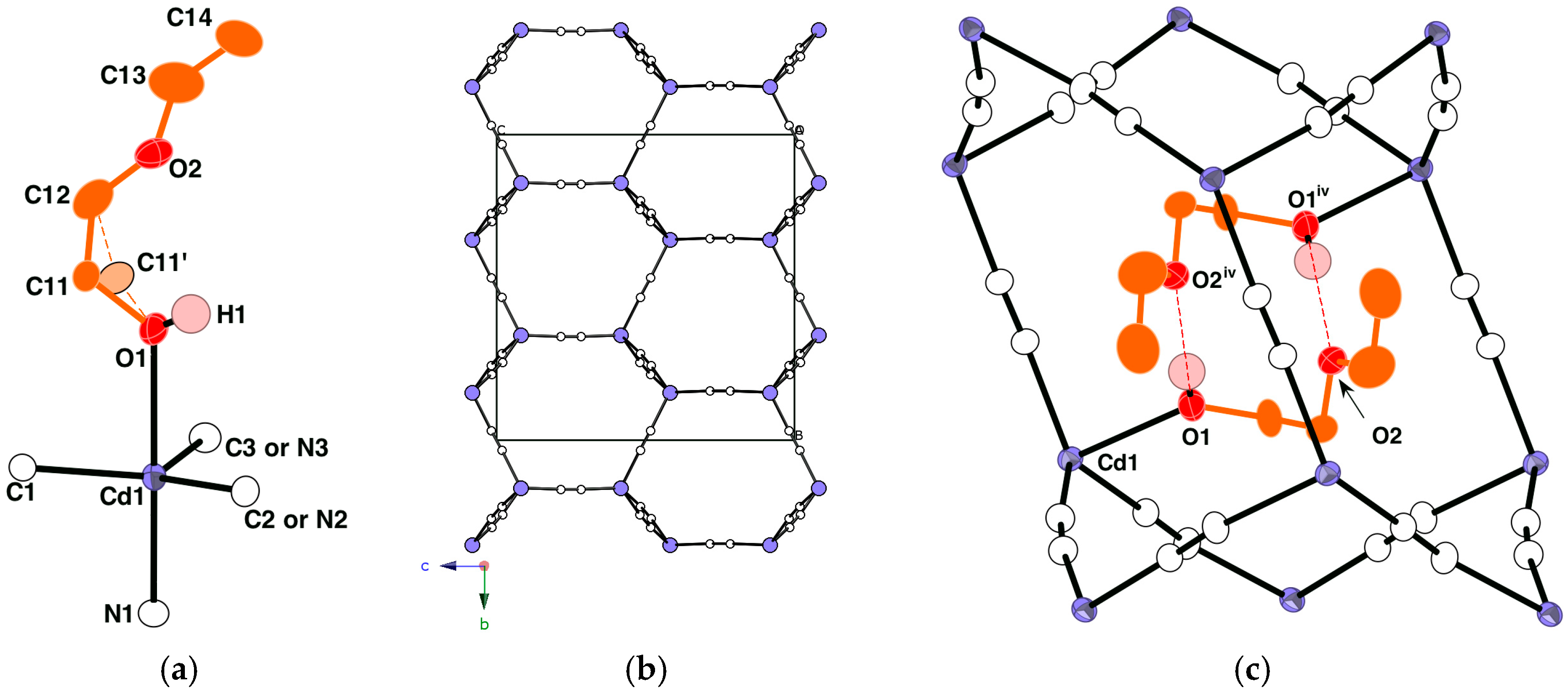
Crystals | Free Full-Text | Synthesis and Crystal Structures of Cadmium(II) Cyanide with Branched-Butoxyethanol

Homologous Critical Behavior in the Molecular Frameworks Zn(CN)2 and Cd (imidazolate)2 | Journal of the American Chemical Society
Assertion : In a mixture of Cd(II) and Cu(II), Cd^2+ gets precipitated in presence of KCN by H2S. - Sarthaks eConnect | Largest Online Education Community
![The crystal structure of [Cd(4-ethylpyridine) 2 ][Au(CN) 2 ] 2 : (a)... | Download Scientific Diagram The crystal structure of [Cd(4-ethylpyridine) 2 ][Au(CN) 2 ] 2 : (a)... | Download Scientific Diagram](https://www.researchgate.net/publication/299405240/figure/fig2/AS:668676856500225@1536436394135/The-crystal-structure-of-Cd4-ethylpyridine-2-AuCN-2-2-a-the-local-structure_Q320.jpg)
The crystal structure of [Cd(4-ethylpyridine) 2 ][Au(CN) 2 ] 2 : (a)... | Download Scientific Diagram
Crystals | Free Full-Text | Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol

Kniha Fraus Ilustrovaný studijní slovník NČ-ČN +CD ROM (2.vyd.) | Odborná literatúra a právnická literatúra SPRINTON

H2S is bubbled into a 0.2 M NaCN solution which is, 0.02 M each in Ag(CN)2^ and (Cd(CN)4)^2 - . If Ksp of Ag2S and CdS are 10^-50 and 7.1 x 1O^-28

Calculated total DOS and PDOS of (a) Cd ( CN ) 2 and (b) Zn ( CN ) 2... | Download Scientific Diagram
![Cd^2 + and Cu^2 + are separated by complex formation using KCN in which K3 [ Cu (CN)4 ] is more stable than K4 [ Cd (CN)4 ] ; on passing H2S Cd^2 + and Cu^2 + are separated by complex formation using KCN in which K3 [ Cu (CN)4 ] is more stable than K4 [ Cd (CN)4 ] ; on passing H2S](https://d1hj4to4g9ba46.cloudfront.net/questions/203099_8b7eea5a5e2c463b942ff76d761e33c3.png)



![Answered: For the aqueous [Cd(CN)4]^2- complex… | bartleby Answered: For the aqueous [Cd(CN)4]^2- complex… | bartleby](https://content.bartleby.com/qna-images/answer/35795836-fd88-45f1-985d-7308e4b8ab78/48218067-d5cd-419c-be2e-3bfd7639ce3c/yyl70ip.png)



