vant hoff theory of dilute solution | van't hoff | solution | chapter 2 | chemistry | class 12th - YouTube
![What is expected value of van\'t Hoff factor for `K_(3)[Fe(CN)_(6)]` in the dilute solution? - YouTube What is expected value of van\'t Hoff factor for `K_(3)[Fe(CN)_(6)]` in the dilute solution? - YouTube](https://i.ytimg.com/vi/Uuw7nJ7YZl8/maxresdefault.jpg)
What is expected value of van\'t Hoff factor for `K_(3)[Fe(CN)_(6)]` in the dilute solution? - YouTube
![what are the van't haff factor for the following ?NaHSO4KF4[Fe(CN)6]KNO3Na2SO4NaClplzzzzzzzzz Explain step - Brainly.in what are the van't haff factor for the following ?NaHSO4KF4[Fe(CN)6]KNO3Na2SO4NaClplzzzzzzzzz Explain step - Brainly.in](https://hi-static.z-dn.net/files/d8d/a88860615a09fd5f7597e4ca5e5accf2.jpg)
what are the van't haff factor for the following ?NaHSO4KF4[Fe(CN)6]KNO3Na2SO4NaClplzzzzzzzzz Explain step - Brainly.in





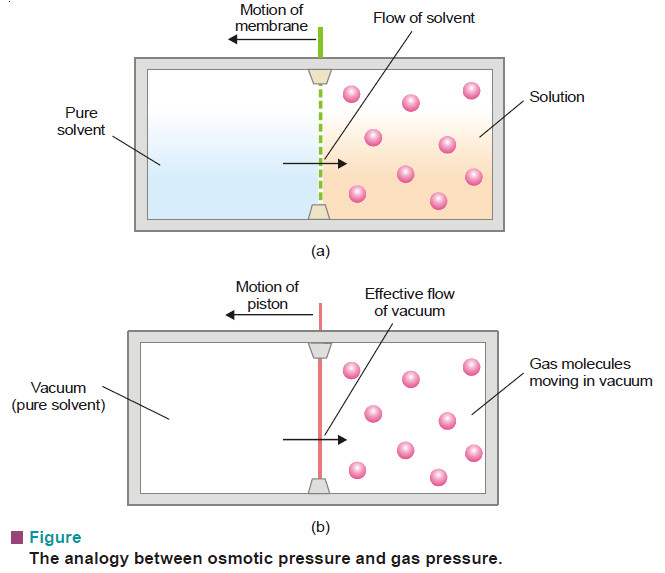



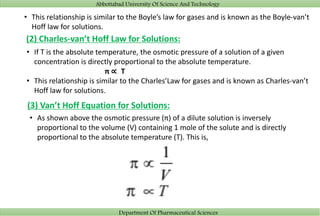
(1)_1595335655_5f16e3e70da0c_391835-10.jpg)